|
Identifying Circadian Output Circuits in Drosophila
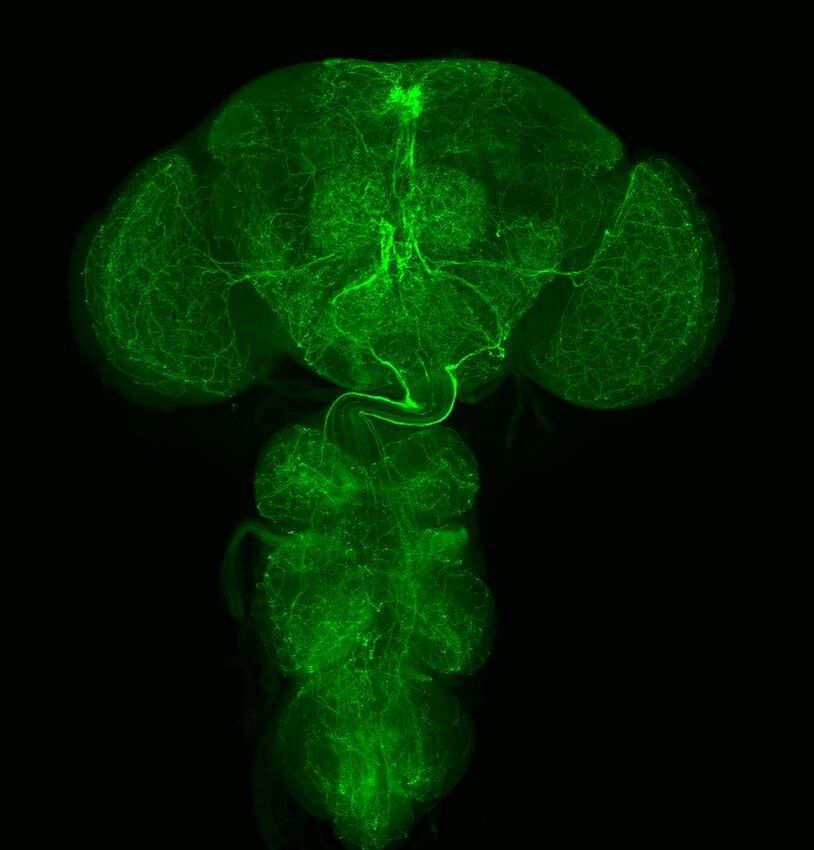
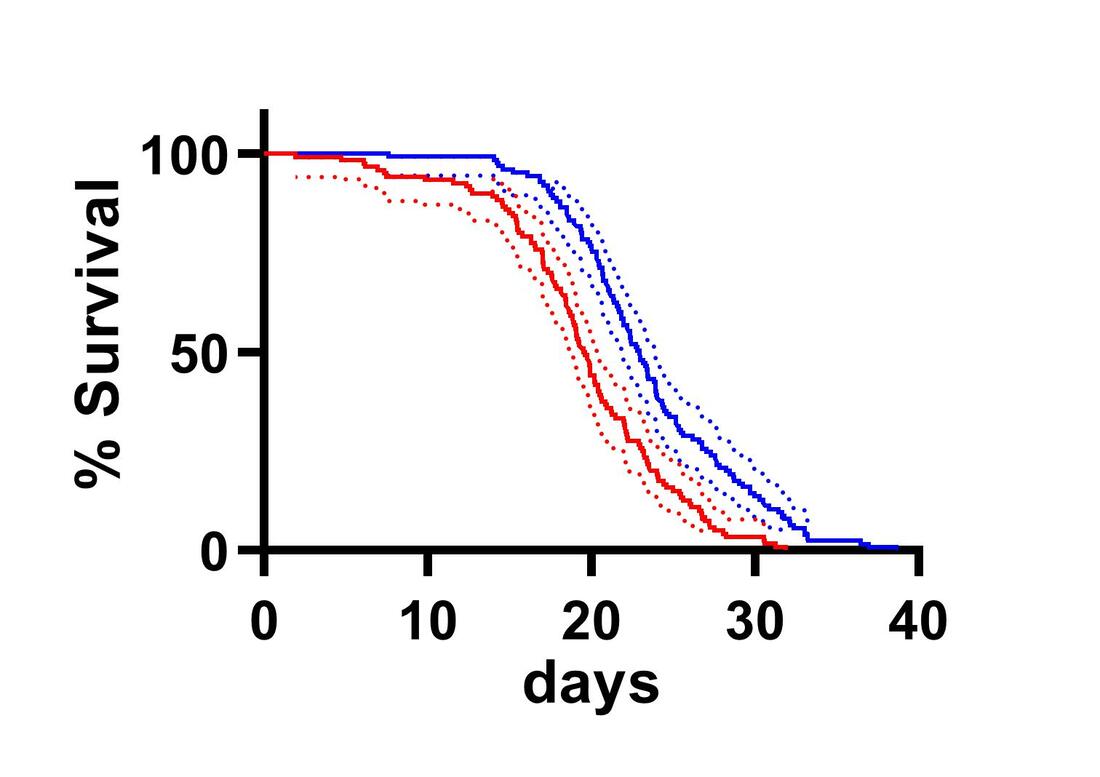
Research in the Cavanaugh Laboratory uses the powerful model organism of the fruit fly, Drosophila melanogaster, to understand the neurobiological basis of circadian rhythms. We take a multifaceted approach, combining state-of-the-art genetic, neuroanatomical, and behavioral techniques, including thermogenetic activation and inactivation of neurons, live functional imaging of neuronal activity, single-cell RNA sequencing, immunohistochemistry, and confocal microscopy, to identify genes, molecules, and neuronal circuits that allow the circadian clock to control diverse physiological and behavioral outputs. The circadian system can be divided into three components: a central clock for timekeeping, input pathways that transmit environmental signals to the clock, and output pathways that couple the clock to behavioral and physiological outputs. These components form a complex network of cells and circuits that together generate rhythms. Though much is known about the identity of core clock neurons and the molecular timekeeping mechanism, fundamental questions remain about the ways in which the circadian clock cells control behavioral and physiological outputs. For example, what are the neuronal circuit mechanisms through which the circadian clock connects to multiple distinct behavioral outputs? What are the genes and molecules used by neurons of the output circuit to control behavioral and physiological outputs? How do cells of the output circuit, which often lack molecular clocks, transmit circadian information? Previous work in the lab has identified the Drosophila pars intercerebralis (PI), the functional equivalent of the mammalian hypothalamus, as an integral component of the circadian output circuit controlling rest:activity rhythms. The PI can be subdivided into multiple neuronal subclasses based on the selective expression of neuropeptides, and we are interested in determining how these different populations contribute to various circadian controlled behaviors, including locomotor activity and feeding. We also seek to understand how information flows between circadian clock cells to coordinately control these output cell populations. In a separate set of studies, we are investigating the consequences of long-term circadian disruption. It is becoming increasingly clear that circadian misalignment, such as occurs in shift workers or as a result of aberrant sleeping and eating schedules common to modern society, has profound metabolic and cognitive consequences. We have developed a model of circadian misalignment in the fly and are using it uncover molecular and physiological mechanisms through which circadian disruption results in reduced organismal health. |
|